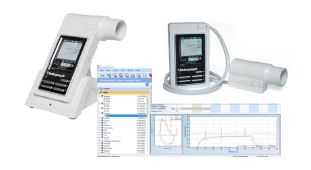
Hand-held In2itive Spirometer with Spirotrac V Software and USB Cradle
Features and Benefits
- Printing with Vitalograph Reports Software (included)
- Miniaturized version of the proven Vitalograph Fleisch pneumotachometer
- Unique optionally removable flow head which allows pneumotach to be held by patient and device to be held by technician
- Tests include FVC (pre and post), VC (SVC), Lung Age, large selection of reportable parameters
- Rechargeable battery operation
- On screen real-time graphs and animated incentive displays
- Can alternate pre and post testing from different patients for rapid test throughput
- Stores up to 10,000 patients in non-volatile memory, backup to Micro-SD
- Complete with protective carrying pouch
Spirotrac Software Features
- Intuitive user interface with workflow-orientated presentation
- Subject list, test session list, dynamic information panel and session data in one view
- Automatic FEV1 trend chart view as subject is selected
- Open session, f/v & v/t curves, all test data, test quality and subject demographics in one view
- Automatic storage of all test data and events, including calibration log
- Attractive incentive displays for children, designed to maximise interest and effort
- View real-time curves through the incentives
- Comprehensive reports for spirometry and trending including PDF generator for remote electronic filling and e-mailing
- Configurable spirometry reports
- Manual data entry and data export facility
- Mannitol/Bronchialitol/Bronchial Challenge Testing
Materials Included
- Hand-held spirometer
- USB cradle
- Vitalograph Reports Software
- Spirotrac V Software, which allows full bidirectional synchronization of patients and data
- Protective carrying pouch
Screen Shots
Technical Specifications
- Subjects: Automatic population of database from Spirotrac. Name, ID, Age, Height, Gender, Smoking Status, Body Mass Index and more depending on model. Storage of up to 10,000 subjects
- Environmental Data: Selectable, Temperature (built in sensors), Barometric Pressure, Altitude, Humidity
- Test Types: Single breath tests, flow/volume loops, multi-breath testing, tidal breathing and combined VC/FVC type test methods supported
- Parameters Measured (depending on model): VC, IVC, IC, VT (TV), TLC, RV, IRV, ERV, FRC, FVC, FIVC, FEV1, FEV3, FEV6, FEV1/VC, FEV1/FVC, FEV3/VC, FEV3/FVC, EV1/FEV6, FEF75, FEF50, FEF25, FEF25-75, FEF25-75/FVC, FIV1, PIF, FIV1/FIVC, FIF25, FIF50, FIF75, FEF50/FIF50, FET, MVVind, FEV1 Ratio, FEV0.5, PEF L/min, PEF L/sec, FEF 0.2-1.2, FEF75-85%, FEF25%, FEF50%, FEF75%, FMFT, FIF25%, FIF50%, FIF75%, PIF, FEV0.75, Lung Age and more
- Predicted Normals: NHANES III, Polgar, Knudson, Crapo, Hsu and many more
- Suggested Interpretation: User Selectable
- Flow Technology: Fleisch Pneumotachograph
- Resolution: 10 mL volume, 0.01 L/s flow
- Data Storage: Stores up to 10,000 subjects and hundreds of test sessions
- Accuracy when in Operating Range:
- Volumes: Better than +/- 3% (0 – 10 L)
- Flows: Better than +/- 10% (0.02 – 16 L/sec)
- Linearity: +/- 5% in range 0.1 to 16 L/s
- PowerSAFETM:
- Input 100 – 240V AC 50-60Hz
- Output – 5V DC
- Battery Pack: Rechargeable Lithium Polymer
- Dimensions: 6 1/4” x 4” x 1 3/4” with flowhead attached
- Storage Humidity: 10% to 95%
- Storage Temperature: 32-122oF
- Recommended Operating Range: 63-98oF, Design Limits: 50 – 104oF
- Ambient Humidity: 0% to 99%
- Connectivity: USB 2
- Max Test Duration: 20 sec. FVC, 30 sec. VC
- Performance Standards: ATS/ERS 2005
- Safety Standards: IEC 60601-1:2005
- Medical Safety Standards: Medical Devices Directive 2007/47/EC
- Designed & Manufactured Under: ISO 13485:2003, FDA 21CFR820 CMDR & JPAL
Warranty Information
- 1-Year Warranty